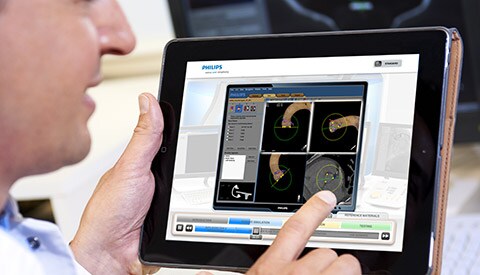
Enterprise deployments of clinical systems and medical devices raise security challenges
Today, many medical devices are designed like specialized computers. Add directives such as Meaningful Use and the desire of clinicians to access patient data from a variety of devices and locations, and it is no surprise that medical devices are occupying an increasing number of nodes on the typical healthcare IT network. As the requirements to ensure patient safety, provide clinicians with convenient access to information and images, and provide medical device security converge, healthcare IT professionals are facing new challenges. Questions include: Learn more about how we can help you easily and securely connect systems, devices and people.